Optimising the Depolymerisation of PET Fleece Microplastics Through Microwave Irradiation
Rya Adronov1
1Westdale Secondary School, Hamilton, ON, Canada
Youth STEM Matters, no. 2 (2022); https://doi.org/10.51892/ysm.2.202203R. Adronov, "Optimising the Depolymerisation of PET Fleece Microplastics Through Microwave Irradiation," Youth STEM Matters, no. 2, 2022.
If you cite this article or use it in any academic activities please let us know by e-mailing us at editor@youthstem2030.org.
Abstract
Introduction
Polyethylene terephthalate (also referred to as polyester, PET, or PETE) is one of the most commonly used plastics worldwide [1]. The polymer is derived from petroleum and formed through the condensation of ethylene glycol and terephthalic acid, with water as a byproduct. The resin formed through this polymerisation is then melted in order to be shaped into various products [2]. PET is extremely versatile in its uses. Its lightweight nature, strength, impermeability, stability at high temperatures, and chemical resistance render the plastic ideal for many applications [3]. The majority of the world’s PET is used in fibres for fabrics and clothing, as well as plastic water bottles. Other uses include food-grade packaging, electrical components, and automotive parts [2, 4].
PET is one of the most recycled plastics, as it is amenable to both mechanical and chemical recycling processes [5]. Mechanically recycled PET is known as rPET, and can be further used in products such as benches, fillers, fleeces, and carpeting [2]. As with most plastics, the mechanical recycling process involves cleaning and melting the used PET to create rPET [6]. This process, however, is not entirely sustainable. Once recycled mechanically, rPET can no longer be reused in the same manner as newly made PET. Studies have shown that with repeated mechanical recycling, rPET loses its mechanical strength and properties, thus becoming less applicable to various uses [7]. To supplement this, rPET is often combined with new PET to create products (typically composed of no more than 50% rPET) [8]. This, however, requires more PET to be made, rendering the original recycling process less impactful.
Figure 1 – Process of polymerisation and depolymerisation.
A promising alternative to these mechanical processes is chemical recycling. This method allows used PET to be returned to its original monomer components through depolymerisation, a reaction that involves chemically breaking down the polymer into its separate constituents (Fig. 1). Depolymerisation can be accomplished through numerous processes, the most widely studied being glycolysis. Glycolysis of PET refers to the process of extensively heating PET in ethylene glycol, using a catalyst (Fig. 2). This process produces bis(2-hydroxyethyl) terephthalate (BHET). The BHET produced can then be re-polymerised to form new PET, rather than rPET [1, 9]. The extensive heating required to perform glycolysis, however, requires substantial amounts of time; upwards of 4 hours is often necessary for depolymerisation to occur, which is both temporally inefficient and uses a relatively substantial amount of energy [10]. Microwave irradiation has recently been shown to be both a more time-efficient - and therefore energy-efficient - process, consequently reducing the cost of PET recycling [1, 10, 11].
Figure 2 – Polymerisation and glycolysis of PET. Terephthalic acid and ethylene glycol polymerise to form PET, with BHET as an intermediate. The reverse reaction is glycolysis.
In 2017, a novel method for chemically recycling PET was reported [1]. In this method, PET is combined with ethylene glycol, a catalyst, and a microwave absorber to form a mixture that is subsequently heated via microwave irradiation, depolymerising the PET to produce BHET. The use of a microwave absorber, a substance that absorbs radiation and converts it to thermal energy, renders glycolytic depolymerisation through microwave irradiation to be extremely energy-efficient [1]. This patented process has been applied to the recycling of post-consumer PET water bottles.
The research presented here employs the method of glycolytic depolymerisation through microwave irradiation [1], previously applied to water bottles, with various PET fleeces. The research aims to apply and optimise this method of chemically recycling PET bottles to depolymerise PET fleece microplastics, through testing of various catalyst amounts, time, and temperature settings.
Recently, it has been discovered that a substantial proportion of microplastic pollution in bodies of water is a result of microfibres, commonly released through the washing of garments in household washing machines. Wastewater treatment plants are often unable to effectively filter out or recycle microfibres, resulting in environmental contamination [12]. Polyester fleece garments are the source of a large amount of these fibres [13, 14, 15]. The fibres, along with the water used to wash them, are drained into lakes and oceans [16]. The microplastic pollution caused by these processes could have severely harmful effects on marine life and human health. Ingestion of PET fibres have been linked with higher mortality rates among sea life [17, 18]. It has also been discussed that the presence of these fibres in seafood destined for human consumption presents human health concerns [19, 20]. Thus, it is of increasing importance that research be conducted with a focus on preventing microplastics from reaching the environment.
The results of this study concluded that reaction conditions of a 2 mg catalyst loading, a 3 minute irradiation time, and a 250°C reaction temperature are optimal for the depolymerisation of PET fleece via glycolysis. These conditions produced the highest yield of BHET, which in turn signifies the highest yield of recycled PET. With further development, these results could be applied to large-scale recycling of PET fleece microfibres that would otherwise contaminate bodies of water worldwide.
Methods
The following procedure was adapted from the patented method for PET water bottle depolymerisation [1]. This method was modified to suit available resources and optimised for the recycling of PET fleeces.
Preparation of Microwave Samples
Stock solutions of both the microwave absorber (NaCl in water, with a concentration of 250 mg/mL) and the catalyst (KOH in ethylene glycol, with a concentration of 100 mg/L) were prepared. Both the microwave absorber and catalyst were purchased from Sigma-Aldrich. 3 mL of solvent (ethylene glycol, also purchased from Sigma-Aldrich) was introduced to microwave vials, followed by 5 µL of NaCl stock solution; these amounts were kept constant throughout all experimentation. Along with a stir bar, KOH stock solution was then added to each microwave vial, in various amounts: 10 µL, 15 µL, 20 µL, 25 µL, 50 µL, and 100 µL (corresponding to 1 mg, 1.5 mg, 2 mg, 2.5 mg, 5 mg, and 10 mg, respectively). In the case of all tests varying catalyst amount, irradiation length, and temperature, a cut sample of 16 oz/yard anti-pill fleece was introduced. This fleece was purchased from PacCana Enterprises Ltd., and samples had an average mass of 223 ± 10 mg and an average area of 9 cm2. The fleece was added to each microwave vial such that each sample was nearly entirely submerged in the solution. Each vial was capped, crimped, and inserted into the sample compartment of a Biotage Initiator+ microwave reactor.
Experimental Settings
Settings deemed to have significant impact on results, besides the catalyst amount, were the length of time each sample was irradiated and the reaction temperature. The irradiation times investigated were 2, 3, and 4 minutes, and the reaction temperatures were 200, 250, and 300°C. Three different sets of samples were tested, each varying one of these three components. As depicted in Table 1, all catalyst loadings were tested using an irradiation time of 3 minutes and a reaction temperature of 250°C. All irradiation times were tested using a catalyst loading of 25 µL and a reaction temperature of 250°C, and all reaction temperatures were tested using a catalyst loading of 25 µL and an irradiation time of 3 minutes. These settings were selected based on preliminary experimentation, which indicated their viability in PET depolymerisation. The conditions corresponding to each set were, in most cases, used in at least three replicate samples.
Table 1 – Overview of preliminary microwave experiments concerning catalyst loading, irradiation time and reaction temperature.
✖ = Experiment performed with a reaction temperature of 200°C
✓ = Experiment performed with a reaction temperature of 250°C
* = Experiment performed with a reaction temperature of 300°C
Fleece Variations
Following analysis of results from preliminary tests, various fleece types were studied. One sample each of 14 oz/yard and 18 oz/yard anti-pill fleeces were tested using a catalyst loading of 25 µL, an irradiation time of 3 minutes, and a reaction temperature of 250°C, prepared in the same manner as the 16 oz/yard fleece. An additional test was run on a fleece sample that had been washed in a simulation of machine washing, in order to assess the applicability of the developed procedure on fleece microfibres as they appear in the environment. To prepare this sample, approximately 50 cm2 of each fleece type (14 oz/yard, 16 oz/yard, and 18 oz/yard) were placed together in a washbasin filled with approximately 2 L of water and 20 mL of Liquinox detergent. The fleece was soaked overnight, then wrung out by hand into the washbasin. Samples of the resulting liquid were subsequently centrifuged to sediment PET microfibres from the liquid. Approximately 5 mg of fibres were extracted, added to a microwave vial in place of a cut fleece sample, and irradiated with 25 µL of catalyst for 3 minutes at 250°C.
Preparation of HPLC Samples
HPLC analysis was performed using a Waters HPLC instrument equipped with a Phenomenex C16 (50 x 4.6 mm) column and UV detector (with 220 nm filter) to detect the composition of all irradiated samples (as well as a commercial BHET sample), and the quantity of each component. HPLC samples, each containing 2 drops of the microwaved sample and 1 drop of acetic acid in 1 mL of dimethyl sulfoxide (DMSO), were prepared and analysed using the following parameters: sample volume 1 µL; solvent gradient water/acetonitrile (0→100% water over 10 minutes).
Microscopy
A Nikon Eclipse LV100N POL polarisation microscope, equipped with an Infinity 1 colour camera, was used to observe a water sample in which the fleece for the washed sample was soaked, as well as the irradiated washed fleece sample.
Results
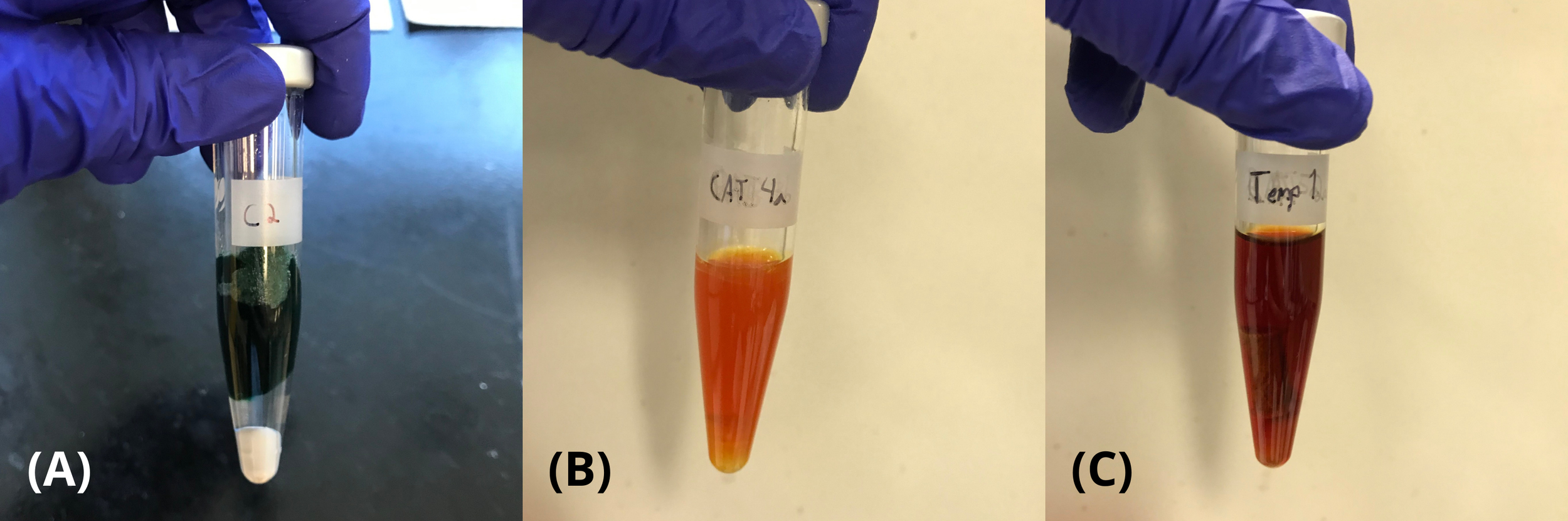
PET fleece samples were irradiated with varying catalyst loadings, irradiation lengths, and reaction temperatures. Post-irradiation samples were inspected both visually and by HPLC to determine the optimal conditions for PET fleece depolymerisation by microwave irradiation. Full depolymerisation of the sample was indicated by the formation of a homogeneous mixture post-irradiation. It was observed that upon irradiation, all resulting samples contained a slightly cloudy, coloured liquid, likely a result of dyes and other additives present in the PET fleeces (Fig. 3). Full degradation of fleece occurred upon irradiation of all samples irradiated at 250°C or above; in these samples, no visible remnant of the original fleece remained (Fig. 3B). It was observed that when the reaction temperature was below 250°C, there was an incomplete degradation resulting in a remnant that resembled a grey, fibreless, brittle version of the fleece that had been inserted (Fig. 3C).
Figure 3 – Samples at varying stages of degradation. (A) 16 oz/yard fleece to be irradiated. (B) Post-irradiation, in which full degradation of the fleece occurred. (C) Post-irradiation, in which the fleece had not fully degraded, resulting in a remnant of fleece.
Figure 4 – The sample containing washed fleece microfibres after irradiation.
Though the colour of fleece used in samples did not impact the process of irradiation, colour changes occurred with each different colour of fleece irradiated. Samples originally containing green fleece produced an orange liquid after irradiation, while samples containing red fleece produced a deep purple liquid. Samples containing blue fleece produced a light pink liquid, and the sample containing washed fleece microfibres produced a pale yellow liquid (Fig. 4). It was noted that with increasing amounts of catalyst, longer irradiation times, and higher reaction temperatures, the liquid became lighter in colour. Small amounts of a white precipitate were observed in most samples and could be clearly seen after they had settled upon standing for several hours in the vial.
Quantitative sample analysis was performed by HPLC. All components of the mixtures eluted after between 0.5 and 5 minutes (Fig. 6). The highest-intensity peak, observed at 3.1 minutes in all chromatograms, was attributed to BHET, the main product of PET depolymerisation (structure shown in Fig. 5). This was confirmed by the injection of a commercial sample of BHET provided by Sigma-Aldrich, which eluted at 3.1 minutes (Fig. 7). Additional significant peaks were observed at 4, and in some cases at 4.5, minutes. These were attributed to the presence of dimer (structure shown in Fig. 5) and trimer structures of BHET, respectively. Furthermore, peaks were observed at 0.7 and 1.2 minutes. The peak at 0.7 remained constant throughout all samples, including the blank, which indicates that the peak does not arise from the sample; rather, it is hypothesised that this peak is a result of the acetic acid added to all HPLC samples. The peak at 1.2 minutes increased in intensity with larger catalyst loadings and higher reaction temperatures. This component may be a terephthalic acid derivative, produced from BHET hydrolysis caused by small amounts of water present in the reaction mixture. However, it was not possible to definitively determine the identity of this component. The HPLC analysis confirmed that BHET was the major product in all samples tested.
Figure 5 – Chemical structures of BHET and BHET dimer.
Figure 6 – HPLC chromatogram of a representative sample showing all peaks observed. This includes a peak at 3 minutes attributed to BHET, the main product in PET depolymerisation.
Figure 7 – HPLC chromatograms. (A) Overlaid HPLC chromatograms of all samples using 2.5 mg of KOH, an irradiation time of 3 minutes, and a reaction temperature of 250°C. (B) HPLC chromatogram of a sample of commercial BHET.
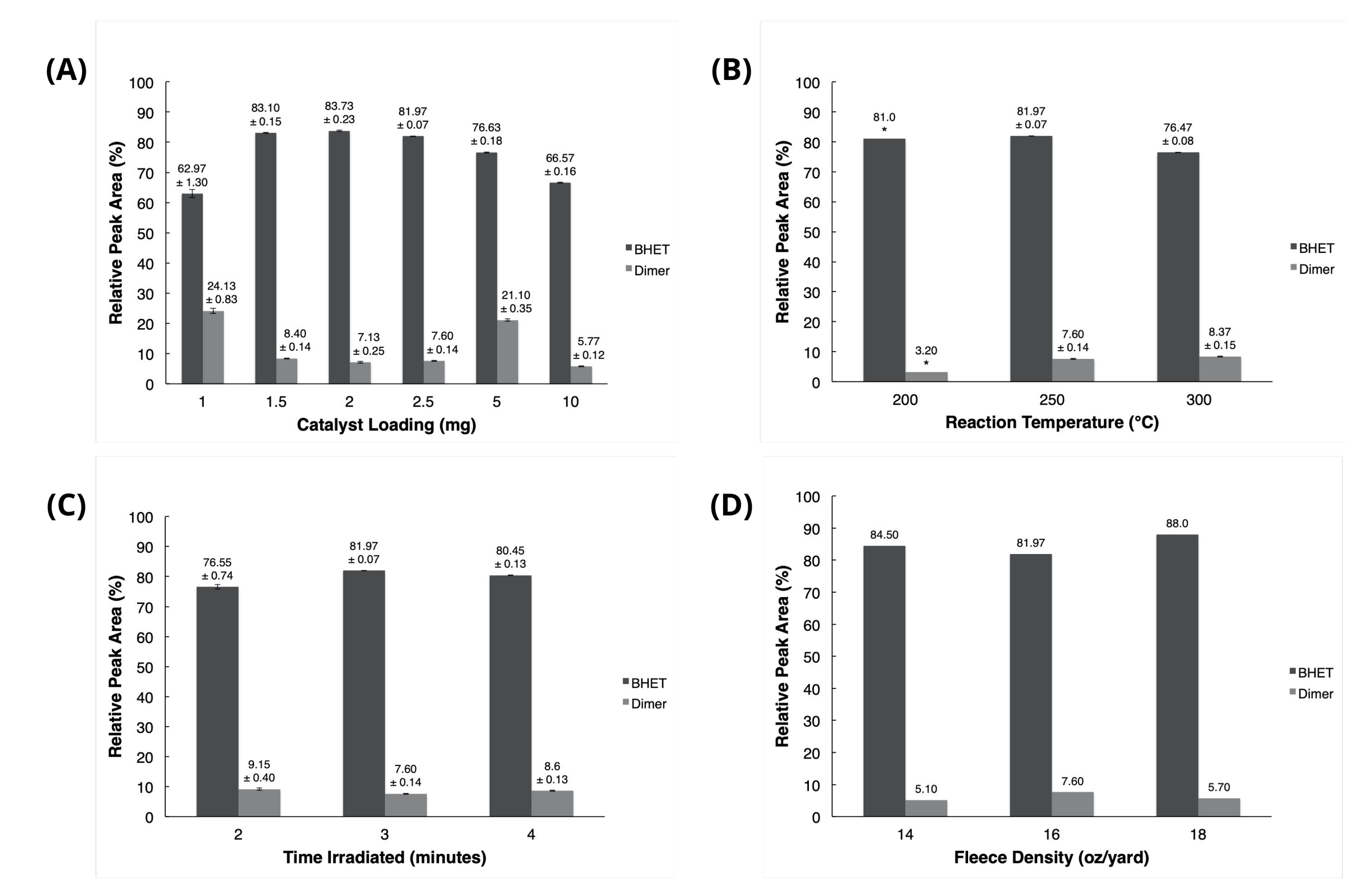
The relative composition of samples was analysed through integration of all significant peaks that eluted after between 1 and 5 minutes. The relative peak areas attributed to BHET and the dimer were recorded and compared. The average percentages and standard deviations of relative peak areas for these specific peaks, indicating the amount of BHET and dimer present in each sample, are presented in Fig. 8.
Figure 8 – Average HPLC BHET and dimer Peak Areas (error bars represent standard deviation) for various: (A) catalyst loadings. (B) reaction temperatures. (C) irradiation times. (D) fleece types.
*Results from the sample irradiated at 200°C in (B) are invalid, due to the observation of a fleece remnant which had not depolymerised during irradiation.
Microscopy
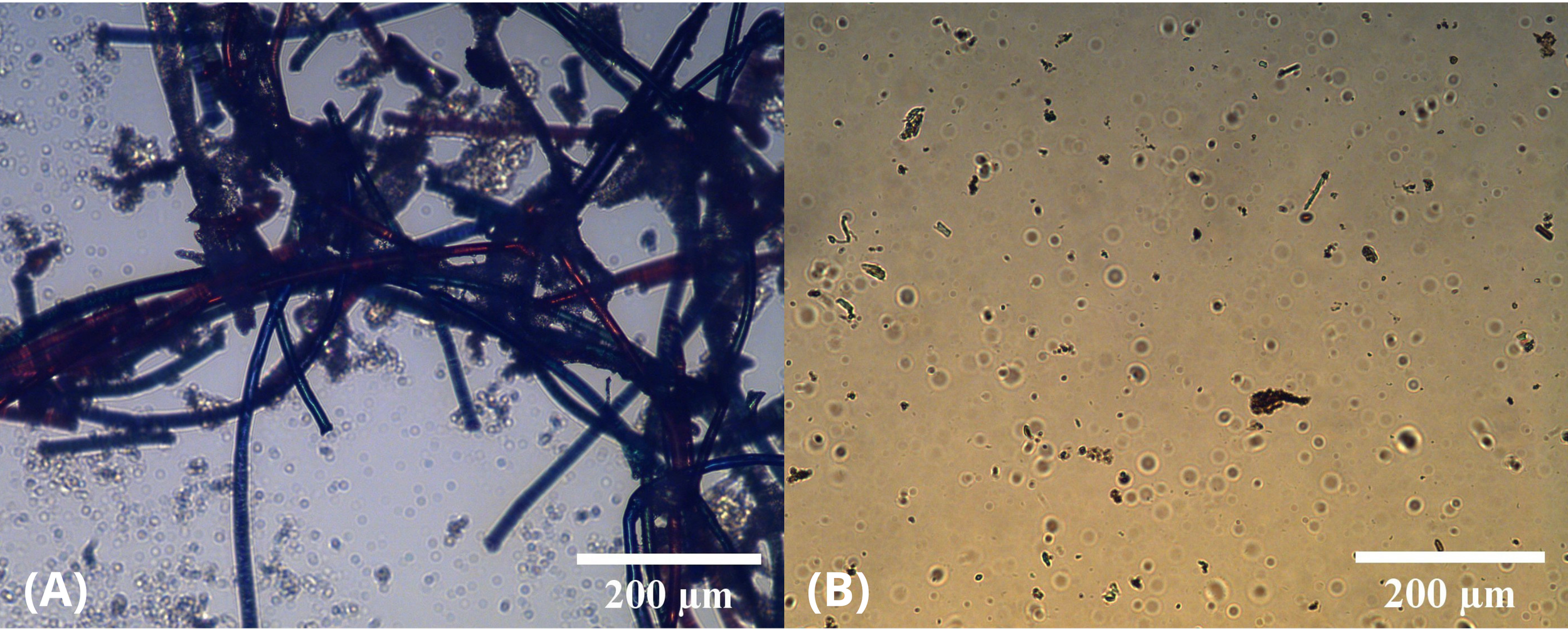
Polarised optical microscopy was performed on a sample of water in which fleece had been soaked overnight, as well as on the post-irradiation washed fleece sample. In the water sample, microfibres of various colours were visible. These microfibres varied in size, from approximately 4 µm to 400 µm in length, and from approximately 30 µm to 80 µm in diameter (Fig. 9A). There was visibly a substantially lower concentration of microfibres in the irradiated sample of washed fleece (Fig. 9B). This confirms that the microfibres were completely degraded as a result of the depolymerisation process, and that depolymerisation of microfibres occurred through irradiation using the presented procedure.
Figure 9 - Microscope images of samples. (A) Water in which fleece was soaked. (B) Irradiated washed fleece.
Discussion
As depicted in Fig. 8A, a catalyst loading of 2 mg (or approximately 1 mass percent relative to the amount of fleece added) resulted in the highest BHET yield, and catalyst loadings above or below 2 mg exhibited either larger amounts of dimer or lower amounts of BHET. Fig. 8B indicates that a reaction temperature of 250°C yields the largest amount of BHET and the lowest amount of dimer of all temperatures tested, as well as that a temperature of 200°C is too low to allow complete degradation of the fleece sample. Fig. 8C shows that an irradiation length of 3 minutes produced more BHET and less dimer than other lengths tested. It can thus be concluded that a catalyst loading of 2 mg, a reaction temperature of 250°C, and an irradiation length of 3 minutes are the optimal conditions for PET fleece depolymerisation. These settings produced relative amounts of BHET upward of 80%, and amounts of dimer below 8%. Different fleece masses tested had little effect on the efficiency of the reaction, as shown in Fig. 8D.
The HPLC chromatograms in Fig. 7 compare relative peak areas of irradiated samples to a sample of commercial BHET. It should be noted that the chromatograms show a notably larger percentage of dimer in the commercial sample when compared to the irradiated samples. Thus, the BHET produced using conditions presented in this report is of comparable or better purity relative to commercial BHET.
In some samples, the fleece did not degrade after irradiation, and instead appeared to be packed into the bottom of the microwave vial. The liquid in these vials appeared lighter than in those irradiated under the same conditions which had fully degraded. It is hypothesised that the stir bar in these vials became stuck and was unable to properly stir the mixture during irradiation. Most of these samples were replicated an additional time, and all such replicates showed results identical to those irradiated under the same conditions which had fully degraded. The samples in which the fleece did not degrade were thus deemed outliers and excluded from all further analyses.
Certain sources of error were identified. The irradiation time was not entirely accurate or precise as the microwave’s timer began when the set temperature had been reached; the resulting variation in the time taken to reach the set temperature means there was a slight inconsistency in the overall irradiation time. Consistency in HPLC peak integration was also a potential source of error, as this process was done manually by starting and ending at the baseline around each peak. Thus, slight differences in exact integration may arise from this manual process from one replicate to the next. Additionally, any peaks that were not fully resolved were difficult to integrate in an accurate and precise manner.
It was found that microwave irradiation in the presence of both a catalyst and a microwave absorber with a reaction temperature above 200°C caused PET fleece samples to fully degrade. This procedure was also applicable to a sample of microfibres isolated from a simulated fleece washing process. The isolated BHET product resulting from irradiated samples had a comparable purity to that of a commercial BHET sample.
Based on the average amounts of both BHET and dimer present in the irradiated samples, it was concluded that a catalyst amount of 2 mg (approximately 1 mass percent relative to mass of fleece), an irradiation time of 3 minutes, and a reaction temperature of 250°C are optimal conditions for PET fleece depolymerisation at the scale investigated.
This research addresses a novel method of recycling fleece microplastics, as a solution to the globally concerning issue of microplastic pollution; specifically of microfibre pollution originating from PET fleece garments [1]. This pollution has a substantial effect on our environment. It is necessary to reduce microfibre pollution present in bodies of water in order to mitigate risks to human health in the future. The work presented here builds on research in the area of recycling PET through microwave irradiation, which has previously been focused on investigating the validity of the method, as well as on the recycling of PET bottles [10, 21, 22, 23]. This work expands on previous research to include optimised glycolysis of PET microfibres, accompanied by the use of a microwave absorber in the irradiation process. Future research would involve further optimisation of the procedure, with a focus on conditions suited to the depolymerisation of washed fleece. The research presented here could be advanced and applied to systems with the goal of recycling PET microfibres before they are released into the environment. This could be achieved, for example, through microfibre filtration and irradiation processes directly in wastewater treatment plants, in order to prevent microfibres originating from washing machines from proceeding to bodies of water. These treatment plants have been determined to be the primary recipients of microplastics prior to their release [24]. However, the technologies employed in these plants are unable to completely remove microplastics from wastewater, and the effectiveness of treatment processes varies between plants [12]. Improving and expanding these processes to incorporate on-site PET recycling could play a significant role in preventing PET microfibres from polluting the environment in the future.
Acknowledgements
The research presented here would not have been possible without the support of the Department of Chemistry and Chemical Biology at McMaster University, which generously provided use of their lab space, materials, and equipment, as well as guidance. Many thanks to Mitchell McLoed and Millipore Sigma-Aldrich, for providing a sample of commercial BHET; to Professor José Moran-Mirabel and Manjot Grewal, for their assistance and advice; to Dr. Matthew Parrott, for his guidance and support; and to Isra Bashir and Nancy Kucic, for their encouragement.
Author's Notes
All figures, or tables, were created by the Author, unless otherwise mentioned in the description provided of said figure.
References
[1] M. Parrott, “Chemical recycling of polyethylene terephthalate by microwave irradiation,” US Patent 10508186B2, December 17, 2019.
[2] Encyclopaedia Britannica, “Polyethylene Terephthalate,” May 2020. [Online]. Available: https://14.britannica.com/science/polyethylene-terephthalate. [Accessed 16 March 2022].
[3] G. L. Robertson, “Food Packaging,” Encyclopedia of Agriculture and Food Systems, vol. 3, pp. 232-249, 2014. Available: https://doi.org/10.1016/B978-0-444-52512-3.00063-2.
[4] C. Crawford and B. Quinn, “Physiochemical properties and degradation,” Microplastic Pollutants, 4, pp. 57-100, 2017. [Online]. Available: https://doi.org/10.1016/B978-0-12-809406-8.00004-9.
[5] T. Chilton, S. Burnley and S. Nesaratnam, “A life cycle assessment of the closed-loop recycling and thermal recovery of post-consumer PET,” Resources, Conservation and Recycling, vol. 54, no. 12, pp. 1241-1249, 2010. Available: https://doi.org/10.1016/j.resconrec.2010.04.002.
[6] European Bioplastics, “Mechanical Recycling,” July 2020. [Online]. Available: https://docs.european-bioplastics.org/publications/bp/EUBP_BP_Mechanical_recycling.pdf. [Accessed 18 March 2020].
[7] Y. Srithep, D. Pholharn, A. Dassakorn and J. Morris, “Effect of chain extenders on mechanical and thermal properties of recycled poly (ethylene terephthalate) and polycarbonate blends,” IOP Conf. Series: Materials Science and Engineering, vol. 213, no. 1, pp. 213, 2017. Available: https://doi.org/10.1088/1757-899X/213/1/012008.
[8] E. J. Velásquez, L. Garrido, A. Guarda, M. J. Galotto and C. López de Dicastillo, “Increasing the incorporation of recycled PET on polymeric blends through the reinforcement with commercial nanoclays,” Applied Clay Science, vol. 180, 2019. Available: https://doi.org/10.1016/j.clay.2019.105185.
[9] G. Xi, M. Lu and C. Sun, “Study on depolymerization of waste polyethylene terephthalate into monomer of bis(2-hydroxyethyl terephthalate),” Polymer Degradation and Stability, vol. 87, no. 1, pp. 117-120, 2005. Available: https://doi.org/10.1016/j.polymdegradstab.2004.07.017.
[10] D. S. Achilias, et al., “Glycolytic depolymerization of PET waste in a microwave reactor,” Applied Polymer Science, vol. 118, no. 5, pp. 3066-3073, 2010. Available: https://doi.org/10.1002/app.32737.
[11] T. Razzaq and C. O. Cappe, “On the Energy Efficiency of Microwave-Assisted Organic Reactions,” ChemSusChem, vol. 1, no. 1-2, pp. 123-132, 2008. Available: https://doi.org/10.1002/cssc.200700036.
[12] T. Zhang et al., “A review of the removal of microplastics in global wastewater treatment plants: Characteristics and mechanisms,” Environment International, vol. 146, 2021. Available: https://doi.org/10.1016/j.envint.2020.106277.
[13] K. Vassilenko, M. Watkins, S. Chastain, A. Posacka and P. S. Ross, “Me, my clothes and the ocean: The role of textiles in microfiber pollution,” 2019. [Online]. Available: https://assets.ctfassets.net/fsquhe7zbn68/4MQ9y89yx4KeyHv9Svynyq/8434de64585e9d2cfbcd3c46627c7a4a/Research_MicrofibersReport_191004-e.pdf. [Accessed 18 March 2020].
[14] U. Pire, M. Vidmar, A. Mozar and A. Kržan, “Emissions of microplastic fibers from microfiber fleece during domestic washing,” Environmental Science and Pollution Research, vol. 23, pp. 22206-22211, 2016. Available: https://doi.org/10.1007/s11356-016-7703-0.
[15] I. E. Napper and R. C. Thompson, “Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions,” Marine Pollution Bulletin, vol. 112, no. 1-2, pp. 39-45, 2016. Available: https://doi.org/10.1016/j.marpolbul.2016.09.025.
[16] S. A. Carr, J. Liu and A. G. Tesoro, “Transport and fate of microplastic particles in wastewater treatment plants,” Water Research, vol. 91, pp. 174-182, 2016. Available: https://doi.org/10.1016/j.watres.2016.01.002.
[17] S. Wright, R. Thompson and T. Galloway, “The physical impacts of microplastics on marine organisms: A review,” Environmental Pollution, vol. 178, pp. 483-492, 2013. Available: https://doi.org/10.1016/j.envpol.2013.02.031.
[18] S. C. Gall and R. C. Thompson, “The impact of debris on marine life,” Marine Pollution Bulletin, vol. 92, no. 1-2, pp. 170-179, 2015. Available: https://doi.org/10.1016/j.marpolbul.2014.12.041.
[19] L. Barboza, A. Vethaak, B. Lavorante, A. Lundebye and L. Guilhermino, “Marine microplastic debris: An emerging issue for food security, food safety and human health,” Marine Pollution Bulletin, vol. 133, pp. 336-348, 2018. Available: https://doi.org/10.1016/j.marpolbul.2018.05.047.
[20] M. Smith, D. Love, C. Rochman and R. Neff, “Microplastics in Seafood and the Implications for Human Health,” Current Environmental Health Report, vol. 5, pp. 375-386, 2018. Available: https://doi.org/10.1007/s40572-018-0206-z.
[21] M. Azeem, M. B. Fournet and O. A. Attallah, “Ultrafast 99% Polyethylene terephthalate depolymerization into value added monomers using sequential glycolysis-hydrolysis under microwave irradiation,” Arabian Journal of Chemistry, vol. 15, no. 7, 2022. Available: https://doi.org/10.1016/j.arabjc.2022.103903.
[22] A. Kržan, “Poly(ethylene terephthalate) glycolysis under microwave irradiation,” Polymers for Advanced Technologies, vol. 10, no. 10, pp. 603-606, 1999. Available: https://doi.org/10.1002/(SICI)1099-1581(199910)10:10<603::AID-PAT914>3.0.CO;2-V.
[23] N. D. Pingale and S. R. Shukla, “Microwave-assisted aminolytic depolymerization of PET waste,” European Polymer Journal, vol. 45, no. 9, pp. 2695-2700, 2009. Available: https://doi.org/10.1016/j.eurpolymj.2009.05.028.
[24] J. Sun, X. Dai, Q. Wang, M. C. M. van Loosdrecht and B. Ni, “Microplastics in wastewater treatment plants: Detection, occurrence and removal,” Water Research, vol. 152, pp. 21-37, 2019. Available: https://doi.org/10.1016/j.watres.2018.12.050.
[25] S. Tsubaki, K. Oono, A. Onda, K. Yanagisawa and J. Azuma, “Microwave-assisted hydrothermal hydrolysis of cellobiose and effects of additions of halide salts,” Bioresource Technology, vol. 123, pp. 703-706, 2012. Available: https://doi.org/10.1016/j.biortech.2012.07.086.
About This Article
Peer review information: Primary Handling Research Editor: Shalini Sellam. Coordinating Research Editor: Elizabeth Bourn. Youth STEM Matters thanks Ruvarshe Nyabando, Saanika Mahashetty, Gwyneth MacDonough, Deborah Smith and Lucy Hargreaves for their contribution to the peer review of this work.
Received: 3 January 2022
Accepted: 26 July 2022
Published: 22 September 2022