The Story of DNA Discovery
Deoxyribonucleic Acid in charcoal by Moses Joy Onohoomhen, Youth STEM Matters Artist
In 1958, Francis Crick published the paper that changed our view of modern biology: ‘The Central Dogma of Molecular Biology’. Published in the prestigious journal, Nature, it described the transfer of information from DNA to protein via an RNA intermediate. It stated that ‘such information cannot be transferred back from protein to either protein or nucleic acid’ [1]. Owing to the advancement of scientific technologies and the development of our understanding of DNA, The Central Dogma of Molecular Biology has been revisited and revised, becoming more and more complex with many exceptions and alternative molecular pathways. The central dogma describes the flow of genetic information, but the question remains: “How did we get here?”
Miescher (1869)
The initial idea that there was a sticky substance in nuclei containing information came to be in 1869 when the Swiss physician Friedrich Miescher noticed some peculiar molecules in leukocytes found in pus on used bandages. He observed a substance that precipitated from the solution upon acidification, bearing different characteristics compared to known proteins, which he termed “nuclein” [2]. In fact, his experiment was the first recorded crude purification of DNA. Through biochemical experiments, Miescher was able to determine that this “nuclein” contained carbon, nitrogen and hydrogen (like proteins) but had a high concentration of phosphorus with no detectable sulphur. His work was revolutionary at the time, and he proposed that the nuclein might be the basis of heredity. Getting his work to be accepted into the scientific community was a long and slow process but eventually it was published [3].
Griffith (1928)
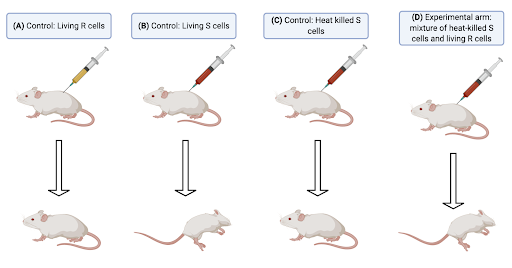
Following Miescher’s discovery, there was a 70-year stagnation in research related to DNA. Finally, in 1928, British bacteriologist Frederick Griffith demonstrated bacterial transformation, a phenomenon in which a bacterium changes its phenotype through the action of a transforming principle [4]. His work involved the use of Streptococcus pneunomiae which has two phenotypic forms: the non-pathogenic, R (rough colonies) and the highly pathogenic, S (smooth colonies) (Fig. 1).
Figure 1: The Griffith Experiments. (A) First control group – a mouse was injected with living R cells and it remained healthy. (B) Second control group - a mouse was injected with living S cells and it contracted pneumonia. (C) Third control group - a mouse was injected with heat-killed S cells and it remained healthy. (D) Experimental arm – a mouse was injected with a mixture of heat-killed S cells and living R cells and it contracted pneumonia. Diagram constructed by author, using Biorender, based on the experiment outlined in Ref [4].
When living R cells were injected into the mouse (Fig. 1A), the R cells could be extracted from cardiac tissue in the mouse but they did not cause pneumonia, hence, they were non-pathogenic. The S cells (Fig. 1B), on the other hand, survived in the mouse and resulted in pneumonia. In contrast, when the pathogenic S cells were killed by heat (Fig. 1C), there was no living Streptococcus pneunomiae that could be extracted from cardiac tissue, and the mouse did not contract pneumonia. The mouse contracted pneumonia when heat-killed S cells and living R cells were injected into it (Fig. 1D), and a mixture of R and S cells could be extracted from the cardiac tissue. This tells us that an S cell transforming principle that survives heat treatment changed some of the R cells, indicating that there was some form of transferrable genetic material present within the cells.
Griffith published his results but the scientific community did not believe him at the time. In fact, he lost his renowned status. We now know that this genetic material is DNA, but it was not until further experiments were carried out by Avery, McLeod and McCarty, as well as Hershey and Chase, that further evidence of the exact nature of the transforming principle came to light [5, 6]. Even then, Avery et al. were not believed either due to popular opinion, which favoured protein as the hereditary material, or because of the 1930 experimental approach.
The tetranucleotide model of DNA
Despite the evidence generated by Griffith in 1928 and Avery et al. in the 1940s, scientists did not believe that DNA could be the hereditary unit due to a tetranucleotide model which was proposed by Phoebus Levene in 1930 [7]. Nevertheless, Phoebus did play a pivotal role in alluding the structure of DNA by citing, using chemical techniques, the monomeric unit of DNA – the nucleotide (Fig. 2A). He then hypothesised this tetranucleotide model which consisted of four nucleotides occuring in tetranucleotide blocks, with the bases pointing outwards (Fig. 2B). This model led Phoebus to the conclusion that DNA was not complex enough to code for the many characteristics that we hold. Consequently, the work conducted by Griffith and Avery et al. was overlooked.
Figure 2 – Levene Conclusions. (A) The structure of a nucleotide. (B) The tetranucleotide model of DNA. B = Base pairs (adenine, guanine, cytosine or thymine). Diagram constructed by author, adapted from Ref [8].
Chargaff’s rules (1950)
In 1944, having read Avery’s report, which stated that the hereditary units were in fact DNA, Erwin Chargaff was determined to better understand the chemistry of nucleic acids. He also realised that if the tetranucleotide model was correct, then the four nucleotides (A, G, C and T) in DNA would be present in equal proportion. Chargaff isolated DNA from different organisms and measured the levels of each of the four nitrogenous bases. Whilst he expected equal values based on the tetranucleotide model, he actually found that the percentage of guanine units equalled the percentage of cytosine units and the percentage of adenine units equalled the percentage of thymine units [9]. He also found that the percentage of G and C varied within the organism, which formed the basis of Chargaff’s Rules, and disproved the tetranucleotide model proposed in 1930. Although he was unable to elucidate the structure of DNA, Chargaff played a crucial role in helping others to determine it.
DNA Structure
We now know that DNA is a linear polymer of nucleotides that adopts a double helix structure in space (Fig. 3) as proposed by Watson and Crick in 1953. They made the model using cardboard and metal scraps. Watson suggested the idea of a specific base pairing scheme (building onto Chargaff’s Rules) and Crick proposed the antiparallel strands. However, neither would have managed to come to these conclusions without the work done by Rosalind Franklin and her famous Photo 51.
Figure 3 – Watson-Crick Model of DNA. LHS: Double helix antiparallel strands of two nucleotide polymers. RHS: A single nucleotide polymer chain, showing polarity. Diagram constructed by author, adapted from Ref [10].
From years of experimental data and evidence, the structure of DNA is well understood and contains several key features (Fig. 3). To begin with, there are two polynucleotide polymer chains wound into a right-handed double-helix wherein the chains are antiparallel (which was later confirmed by Arthur Kornberg in 1961) [11]. Secondly, sugar phosphates are on the outside of the double helix and bases are orientated towards the central axis. There is also complementary base pairing consisting of weak hydrogen bonds between the bases . Furthermore, bases are 0.34nm apart and one complete turn of the helix contains 10.5 bases (initially thought to be 10) since the helix turn is 3.6nm (initially thought to be 3.4nm). Finally, the sugar phosphate backbones are not equally spaced, which results in major and minor grooves. To understand how we know this, it is important to examine Rosalind Franklin’s work and her Photo 51.
Rosalind Franklin – Photo 51
James Watson, Francis Crick and Maurice Wilkins proposed the double helix model which we now know to be correct, but it was Franklin who used X-ray crystallography to provide the blueprints behind the discovery. In 1950, Maurice Wilkins attempted to use X-ray crystallography to unveil the structure of DNA but he was unsuccessful. He stretched the DNA and then air dried it, producing an X-ray diffraction of dried DNA (which he called type ‘A’ DNA). His photograph was very complex and made it too difficult to interpret the data, so he was initially unsuccessful in deciphering the structure of DNA (Fig. 4A).
Figure 4 – X-ray diffraction patterns of the A and B forms of DNA. Reproduced from Ref [12].
On the other hand, after many hours of X-ray exposure in 1952, Rosalind Franklin managed to produce ‘Photo 51’ by stretching the DNA and leaving it hydrated, thereby producing a distinct cross structure (Fig. 4B). In the autumn of 1951, Franklin showed some of her data to her students in a lecture at Kings College and James Watson is said to have been in the audience. She was unable to explain the significance of the cross shape in the X-ray photograph, but Watson soon realised that DNA must be helical and told Francis Crick. They then put together a three-helical structure of DNA and showed Rosalind Franklin their model. She said it was utterly wrong and their working relationship went downhill.
Meanwhile, there was some conflict between Maurice Wilkins and Rosalind Franklin while they were both working on the DNA project. It is said that the former was bitter because the latter was to lead the department [13]. In 1953, Watson visited Wilkins and, without Franklin’s permission, Wilkins showed him Franklin’s Photo 51. Watson was astonished by the detail and quickly jotted down a sketch to show Crick. Four weeks later, they had deciphered the structure of DNA [14]. The two invited Franklin to look at the model, and she was very cautious in pointing out that there was not enough evidence to overstate their findings from. She went on to describe their model as ‘very pretty’ then asked them, “But how are you going to prove it?”. Fortunately, now we know that their model is correct, and Franklin played a pivotal role in generating the model. The sad truth is that her work did not receive any official recognition. Nevertheless, her contributions paved the way for the Watson-Crick model of DNA.
DNA in our modern society
The work done by Watson, Crick, Wilkins and Franklin has shaped our modern understanding of genetics and DNA. Our understanding of DNA as a hereditary unit has led to the classification of the human genome via the Human Genome Project. Furthermore, we are able to manipulate DNA and genes providing therapeutic advantages and enhancements in biotechnology and bioinformatics.
References
[1] F. Crick, "Central Dogma of Molecular Biology," Nature, vol. 227, pp. 561-563, 1970.
[2] R. Dahm, "Friedrich Miescher and the discovery of DNA," Developmental Biology, vol. 278, p. 274–288, 2005.
[3] F. Miescher-Rüsch, "Ueber die chemische Zusammensetzung der Eiterzellen," Medicinisch-chemische Untersuchungen, vol. 4, 1871.
[4] F. Griffith, "The Significance of Pneumococcal Types.," The Journal of hygiene, vol. 27, no. 2, pp. 113-59, 1928.
[5] O. T. Avery, C. M. MacLeod and M. McCarty, "Studies on the Chemical Nature of the Substance Inducing Transformation of Pneumococcal Types: Induction of Transformation by a Desoxyribonucleic Acid Fraction Isolated from Pneumococcus Type III," The Journal of experimental medicine, vol. 79, no. 2, p. 137–158, 1944.
[6] A. D. Hershey and M. Chase, "Independent functions of viral protein and nucleic acid in growth of bacteriophage," The Journal of general physiology, vol. 36, no. 1, pp. 39-56, 1952.
[7] I. Hargittai, "The tetranucleotide hypothesis: a centennial," Structural Chemistry, vol. 20, p. 753–756, 2009.
[8] M. Fry, "Dissolution of Hypotheses in Biochemistry: Three Case Studies.," History and Philosophy of the Life Sciences, vol. 38, no. 4, p. 1–40, 2016.
[9] N. Kresge, R. D. Simoni and R. L. Hill, "Chargaff’s Rules: the Work of Erwin Chargaff," Journal of Biological Chemistry, vol. 280, no. 24, pp. 172-174, 2005.
[10] J. M. Berg, J. L. Tymockzo, J. Gregory J. Gatto and L. Stryer, Biochemistry, vol. Eight, New York: W. H. Freeman and Company, 2015.
[11] J. Josse, A. D. Kaiser and A. Kornberg, "Enzymatic Synthesis of Deoxyribonucleic Acid," Journal of Biological Chemistry, vol. 236, no. 3, pp. 864-875, 1961.
[12] R. E. Franklin and R. G. Goslino, "The Structure of Sodium Thymonucleate Fibres. I. The Influence of Water Content," Acta Crystallographica , vol. 6, p. 673, 1953.
[13] M. J. Tobin, "Three Papers, Three Lessons," American journal of respiratory and critical care medicine , vol. 167, pp. 1047-1049, 2003.
[14] J. Watson and F. Crick, "A structure for deoxyribose nucleic acid," Nature, vol. 171, p. 737–738, 1953.
Hello Youth STEM Matters Research Conference attendee! Can you see the impact that scientific collaboration and innovation can have?
The phrase in bold is what you'll need :)

![Figure 2 – Levene Conclusions. (A) The structure of a nucleotide. (B) The tetranucleotide model of DNA. B = Base pairs (adenine, guanine, cytosine or thymine). Diagram constructed by author, adapted from Ref [8].](https://images.squarespace-cdn.com/content/v1/5e2a22a2ca128a771ade8d50/1596119297081-44R9TT89WH57A0QRB84N/Leven+conclusions+diagram)
![Figure 3 – Watson-Crick Model of DNA. LHS: Double helix antiparallel strands of two nucleotide polymers. RHS: A single nucleotide polymer chain, showing polarity. Diagram constructed by author, adapted from Ref [10].](https://images.squarespace-cdn.com/content/v1/5e2a22a2ca128a771ade8d50/1596119490138-FOLOE828SF6TOBXEY66M/Watson-Crick+model+of+DNA)
![Figure 4 – X-ray diffraction patterns of the A and B forms of DNA. Reproduced from Ref [12].](https://images.squarespace-cdn.com/content/v1/5e2a22a2ca128a771ade8d50/1596119547933-KO02UZ8LZGDAYNCT98F9/Rosalind+Franklin%27s+Photo+51)